

Ph.D 1976 Duke University
Postdoctoral work 1977 University of North Carolina
Postdoctoral work 1978 University of Sydney (Queens Fellow)
Assistant Professor 1980;
Associate Professor 1986 University at Buffalo
Professor 1994 University at Buffalo
Chair, Department of Biological Sciences 1999 University
at Buffalo
Mary A. Bisson,
Department of Biological Sciences
623 Hochstetter Hall
State University at Buffalo
Buffalo, NY 14260
(716) 645-4978
Charophytes are macrophytic algae that are taxonomically close to the line that gave rise to higher plants. They have long (up to10 cm) internodal cells that are good subjects for intracellular electrophysiology and intracellular perfusion. We study membrane transport phenomena, especially their regulation. One interest is responses to salt stress. We culture species collected from salt environments (e.g. Chara longifolia from Canada) as well as obligate freshwater algae (e.g. C. corallina from Australia). These species have similar transport systems, but differ in regulatory mechanisms that allow C. longifolia to respond to salt stress whereas C. corallina cannot. To characterize these transport systems, we use cellular techniques such as electrophysiology (whole cell and patch clamping), cellular perfusion and ion flux measurements, as well as molecular techniques to characterize transport proteins. We are also interested in the response to gravity. Statoliths, heavy organelles in the rhizoid, fall in response, but in a complicated and controlled way. We use microscopy and computer analysis techniques to characterize these movements.

Fig. 1. Two species of Chara, C. corallina (left) and C. longifolia (right)
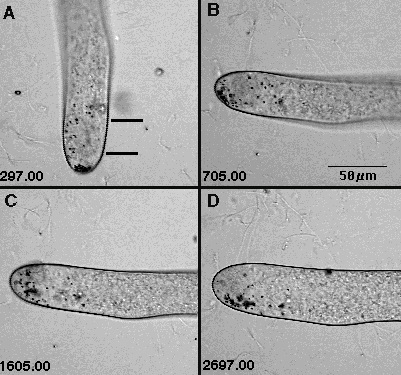
Fig. 2. Movement of statoliths (small dark organelles) in the gravity field. A. Vertical rhizoid. Some statoliths detached and settled; many suspended. 297 s after imaging begun. B. Horizontal rhizoid. Suspended statoliths remain suspended, detached statoliths slide toward bottom. 705 s after imaging begun, 7 min after turning. C. Detached statoliths have been resuspended (defying gravity!). 1605 s after imaging begun, 22 min after turning. D. Both detached and suspended statoliths have begun to settle. 2697 s after imaging begun, 40 min after turning.